Which Are the First Particles to Evaporate From a Liquid
Mass spectrometry MS is an analytical technique that measures the mass-to-charge ratio mz of charged particles ionsAlthough there are many different kinds of mass spectrometers all of them make use of electric or magnetic fields to manipulate the motion of ions produced from an analyte of interest and determine their mz. Is a consequence of 2nd law of thermo.
12 4 Evaporation And Condensation Chemistry Libretexts
Geothermal energy is heat that is generated within the EarthGeo means earth and thermal means heat in GreekIt is a renewable resource that can be harvested for human use.

. At higher temperatures molecules or atoms have a higher average speed and more particles are able to break free of the liquidS surface. Water or various other chemicals may compose the droplets and crystals. Particles sitting at rest.
This transmission is different from what occurs in the aerosol which is a suspension of solid or liquid particles within a gas phase. Specifically the lighter the mass of a decay product the larger the number of possible final states in the kinematical phase space in general. Thus they are very small particles that sediment slowly and are easily conveyed by air currents in this case the transmission is called long.
Vaporization is the process of converting a liquid into a gas. Lets first remind ourselves about what vapor pressure is and what affects it chemically and physically. Once water enters a soil it may leave by percolating to an aquifer being taken up by roots or evaporate directly from the surface.
On the other. We will start with estimation of evaporative flux at steady-state from a water table. The basic components of a mass spectrometer are.
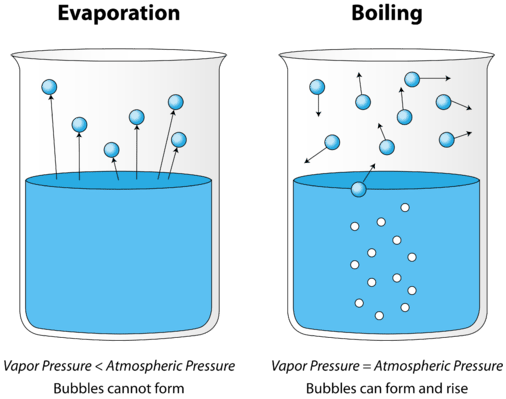
The most common way to add energy to a liquid system is by adding heat. If the water is heated to boiling with big bubbles of vapor popping up from inside the liquid the oil will be less effective in slowing the evaporation. On Earth clouds are formed as a result of saturation of the air when it is cooled to its dew point or when it gains.
For example a wet street will dry. In this section we will quantify the magnitudes of loss through evaporation looking at the impact of atmospheric demand depth to the aquifer and soil texture. For higher boiling point analytes direct immersion SPME is probably necessary.
Direct immersion SPME is warranted for liquid and solution samples which are used in solid-phase and liquid-liquid extraction methods. Higher temperatures also increase the rate of evaporation. The diameter of these particles is normally between 0001 and 100 micrometers.
As a liquid gains energy the molecules begin to move around faster. The coreA small portion of the cores heat comes from the friction and gravitational. However the oil layer can slow the evaporation dramatically.
As this mixture of liquid fuel droplets continues to penetrate into the combustion chamber it entrains more hot air and eventually a point is reached where the rate of energy supplied by the entrained air equals the energy required to evaporate all the fuel emerging from the injector. Talking for 5 minutes and coughing each can produce 3000 droplet nuclei. About 2900 kilometers 1800 miles below the Earths crust or surface is the hottest part of our planet.
Actually the water can evaporate at lower temperatures than 100C just as it could with no oil. At this point the liquid tip. For example the same amount of water will evaporate faster if spilled on a table than if it is left in a cup.
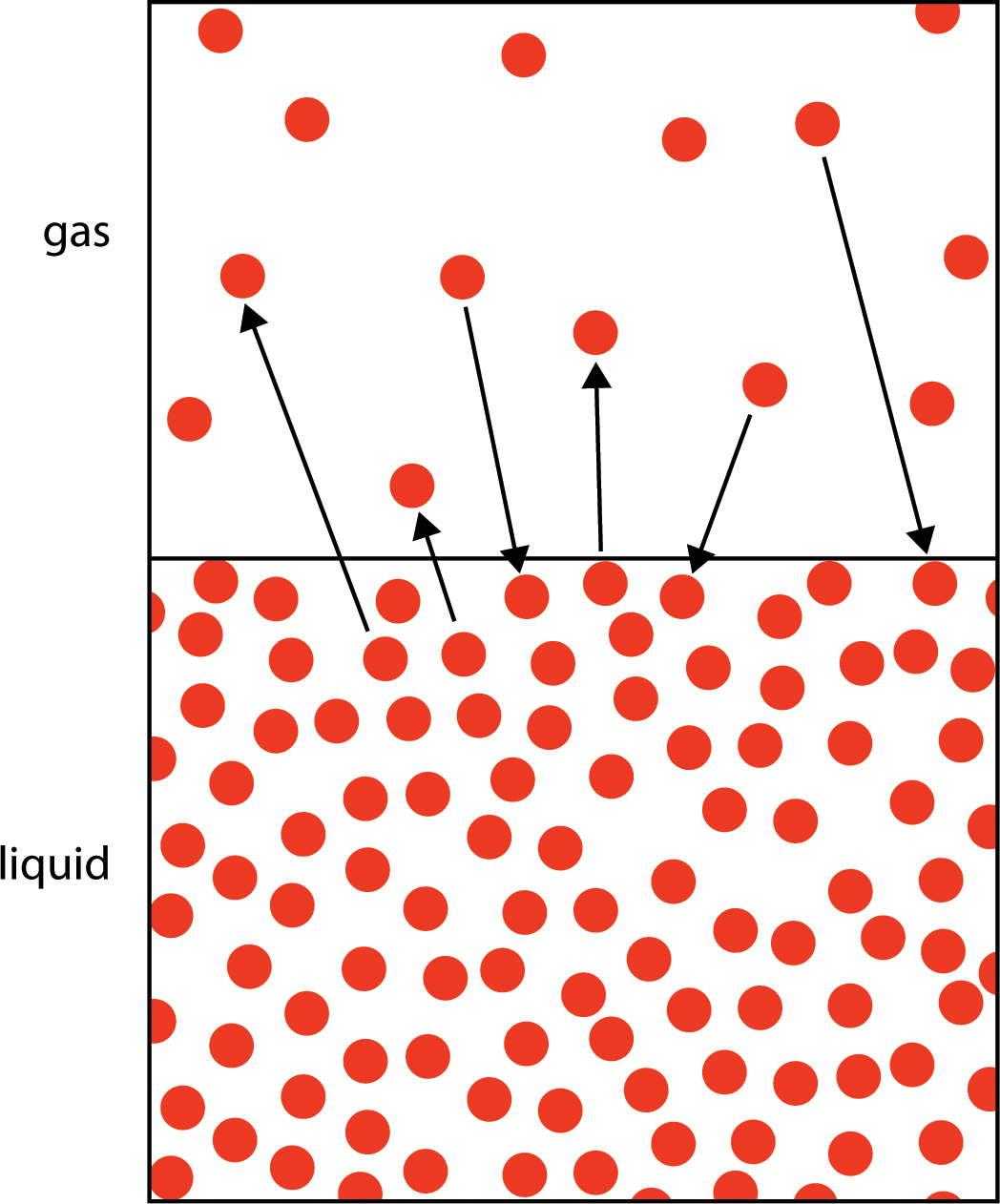
PDF On Nov 24 2014 Sofia Chanioti and others published SolidLiquid Extraction Find read and cite all the research you need on ResearchGate. In a closed container a liquid will evaporate until an equal amount of molecules are returning to the liquid state as there are escaping into the gas phase. It is also called evaporation.
Sneezing can generate approximately 40000 droplets which then evaporate to particles in the size range of 0512 μm. The entrained air heats the liquid fuel and causes it to start to evaporate. The process of nucleation is extremely important in crystallization is the nucleus of a.
The first step in the crystallization process is nucleationThe first atoms in the mass to form a crystal structure become a center and more atoms organize around this nucleusAs this happens more unit cells assemble around the nucleus a small seed crystal is formed. In meteorology a cloud is an aerosol consisting of a visible mass of minute liquid droplets frozen crystals or other particles suspended in the atmosphere of a planetary body or similar space. Headspace SPME is considered for extraction of volatile specious with normal boiling point less than 200C from solid and liquid samples.
For instance if there is just enough energy to make a pair of particles then only one final state is possible. 137 1217 Particles in a biological aerosol usually vary in size from particles may consist of a single unattached organism or may occur in the form of clumps. Since we know that the particles of a gas are moving faster than those of a liquid an input of energy must be required for a liquid to become a gas.
The pressure of the vapor phase above the liquid at this point is called the.
Q What S The Difference Between Evaporation And Boiling Nsta

Evaporation And Condensation Kids Britannica Kids Homework Help


0 Response to "Which Are the First Particles to Evaporate From a Liquid"
Post a Comment